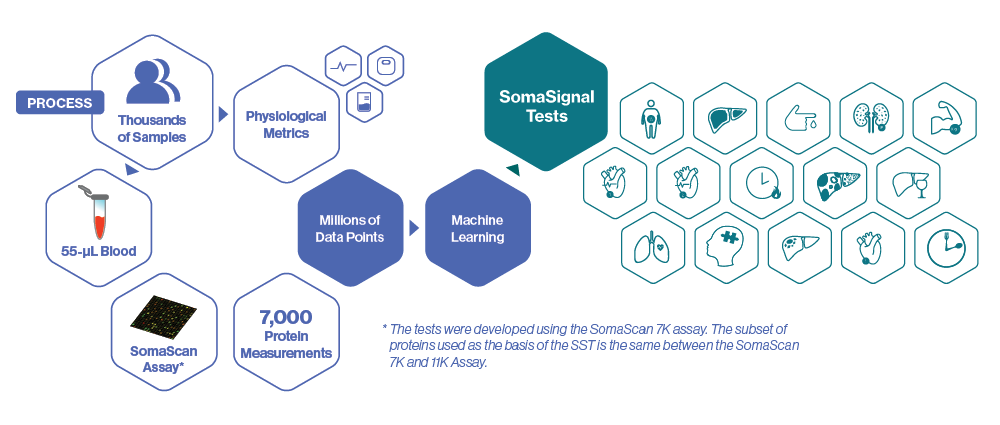
Powerful metrics provide multiple physiological assessments from a single sample
Now available using the SomaScan® 11K Assay
Obtain relevant data to advance your research.
SomaSignal Tests are for Research Use Only, and not to be used for diagnostic or patient medical management purposes. Looking for a diagnostic tool to guide patient medical treatment? Learn about our LDT SSTs.
What are RUO SomaSignal® Tests?
For simple, straightforward clinical trial monitoring
- Simple blood test enables frequent sampling
- Does not require specialized physicians/pathologists
- Simple blood draw is ideal for longitudinal studies
Physiologically meaningful assessments
- 21 SSTs provide multiple metrics from a single sample
- Relevant to cardiovascular disease, NASH, diabetes, and more
- Reporting current health state and understanding risk
Derived from just a 55-µL sample
- Simple and simultaneous sampling for all tests
- Derived from models based on proteomic signatures
- Validated against standard clinical measurement
See the technology
WebinarLiquid health check – plasma protein patterns as comprehensive indicators of health
Earlier this year, Stephen Williams (SomaLogic), Claudia Langenberg (University of Cambridge*), and Peter Ganz (UCSF) lead a team that demonstrated the first proof of concept for a comprehensive “liquid health check” that derived 11 different health indicators from 55µl of blood. The study combined clinical data from five different groups of nearly 17,000 prospectively monitored participants in whom ~5,000 proteins were measured at study baseline using the SomaScan® Assay. Using machine learning models, they found that they were able to characterize six current health states and predict three health related behaviors—equaling metrics that would typically be generated from a battery of costly medical tests and approximately nine visits to the doctor. Additionally, they used the proteomic data and their models to reliably forecast probabilities of cardiovascular events and advancement from prediabetes to diabetes five years later. In this webinar, Williams, Langenberg, and Ganz will discuss key findings and motivations for the study “Plasma protein patterns as comprehensive indicators of health,” which was recently published in Nature Medicine. The webinar will additionally explore the implications of their findings for public health, particularly in the areas of diabetes and cardiovascular disease.WebinarCorrelation of a nonalcoholic steatohepatitis proteomic test with clinical outcomes
In this webinar, Anne Minnich, PhD, biomarker consultant at Bristol Myers Squibb, presents research on the use of the new SomaSignal(TM) NASH bundle test in a recently completed clinical trial.WebinarThe liquid liver biopsy: characterizing NASH and NAFLD with serum protein biomarkers
With SomaScan® Proteomics, predicting NASH and NAFLD is as simple as a blood test. Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) are increasingly common conditions that can progress to serious liver complications including cirrhosis and cancer. Diagnosing the severity of NAFLD and NASH has traditionally relied on invasive liver biopsies. It was recently demonstrated that the SomaScan Assay, a multiplex proteomics platform, can noninvasively and simultaneously predict all the key elements of the liver biopsy: liver fat, inflammation, hepatocyte ballooning and fibrosis based only on serum protein biomarkers. By measuring ~5000 proteins per sample in ~700 patients, with liver biopsy results, machine learning models were developed and validated that can reliably predict NAFLD/NASH phenotypes . The tests were used to characterize the differential mechanistic effects of three different drugs during clinical trials. In this webinar, Dr. Steve Williams, the Chief Medical Officer for SomaLogic, will present this work and introduce a soon to be launched NASH test that is available for research use only through the SomaScan Discovery Alliance.WebinarProteomics reveals the hidden impact of interventions in diabetes
Type 2 diabetes typically manifests itself in adulthood, following years of progressively worsening health status, affecting multiple biological systems, pathways and organs. But beyond measuring HbA1c, it has been difficult and expensive to measure the impact of disease and/or interventions that might impact organ damage or risk. Large-scale proteomics is an emerging field that has recently been shown to not only capture real-time health status, but also to predict or prognose risks of future organ damage, morbidity and mortality. Proteomic models, developed from multiple large clinical and observational studies, have been utilized here to provide a holistic summary of metabolic health and risk of future adverse outcomes from individuals undergoing diabetes intervention. In this webcast, the impact of both therapeutic (drug) and lifestyle (diet and exercise) interventions will be discussed on a host of cardiometabolic and body composition measures in subjects undergoing intervention compared to placebo or standard of care. The speakers will discuss how these proteomic models can be implemented to design more precise treatment strategies for diabetic patients, moving the field ever closer to realizing the goal of providing personalized care.What tests are available for research use?
Body fat %
Percent body fat
Resting energy expenditure
Calories burned at rest
Lean body mass
Total lean body mass
Glucose tolerance
Blood glucose levels following sugar intake
Liver fat
Presence/absence of excess liver fat
Visceral fat
Fat content around vital organs
CVD primary
4-year risk of heart attack, stroke, or heart failure
CVD secondary
4-year risk of heart attack, stroke, or heart failure following an initial event
Cardiorespiratory fitness
Aerobic fitness level (VO2 max)
Alcohol impact
Predicted weekly alcohol consumption
NASH: steatosis
Measures NASH-related liver steatosis
NASH: inflammation
Measures NASH-related liver inflammation
NASH: Fibrosis
Measures NASH-related Liver Fibrosis
NASH: ballooning
Measures NASH-related liver ballooning
Heart Failure reduced Ejection Fraction: 6 months
Risk of death in 6 months with stable HFrEF
Heart Failure reduced Ejection Fraction: 12 months
Risk of death in 1 year with stable HFrEF
Heart Failure preserved Ejection Fraction: 6 months
Risk of death in 6 months with stable HFpEF
Heart Failure preserved Ejection Fraction: 12 months
Risk of death in 1 year with stable HFpEF
Kidney prognosis
Risk of worsening Chronic Kidney Disease
Dementia risk
Risk of Dementia diagnosis within 20 years
Fed-fasted
Hours since last meal
How were they developed?
SomaSignal Tests are developed by analyzing thousands of proteins in thousands of patients using the SomaScan Assay. Protein signatures revealed by the SomaScan Assay are compared against standard clinical measurements in order to identify patterns of protein changes that correlate with current health status and future trajectory using machine learning. Each pattern becomes the basis for a specific SomaSignal Test. Because each SomaSignal Test is generated using a subset of the 11,000 proteins measured by the SomaScan Assay, results for all of the SomaSignal Tests can be derived from a single 55-µL blood sample.