SomaScan technology, with its leading 11K protein measurements, is the only precise proteomic tool
Standard BioTools has achieved a new record of the most protein measurements on the most sample types with expansion of the SomaScan® 11K Platform.
The SomaScan Platform provides the largest number of protein measurements and the greatest number of orthogonally confirmed protein reagents in the proteomics industry. It offers 11,000 protein measurements simultaneously from sample volumes as low as 55 µl—giving researchers access to half of the human proteome in just one assay.
The SomaScan Platform leads the industry with the ability to accurately measure 11,000 proteins. The platform offers low coefficients of variation (CV), with a median around 5%, providing more statistical power to make discoveries. This level of precision ensures that the data generated is reliable, reproducible, and clinically relevant. Without it, even your most promising protein biomarkers could point to misleading conclusions, ultimately affecting patient outcomes.
HIGHEST SCALABILITY
Gain confidence in your longitudinal studies based on unique precision at scale
The SomaScan Platform is the only proteomic approach on the market that successfully scales. The precision of the SomaScan Assay has been maintained or improved across all versions, up to and including the most recent release covering 11,000 protein measurements—a challenge that antibody-based assays have failed to overcome.
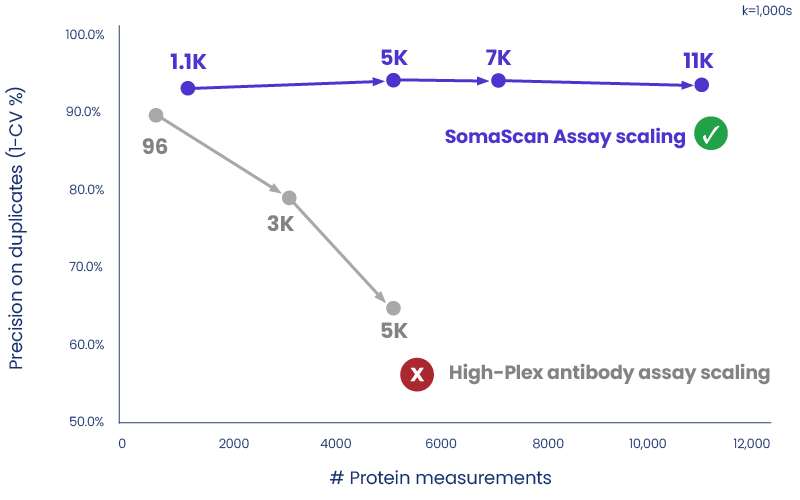
*Source: Rooney, M.R. et al., 2024. Plasma proteomic comparisons change as coverage expands for SomaLogic and Olink. medRXiv.
As SomaScan Assay expands from 5,000 to 7,000 to 11,000 proteins, precision in measurements has been maintained. In contrast, high-plex antibody assays have shown a significant loss in precision as the assay attempts to scale.
Future-proof your longitudinal studies: The SomaScan Assay’s unparalleled precision and data quality process enables direct comparison of studies within and between assay versions, unlike high-plex antibody assays. This means every valuable sample assayed with the SomaScan Platform can be used for study insights, critical to your large-scale studies.
LOWEST CVs
The SomaScan Assay is the industry-leading proteomics platform with the lowest CVs
The SomaScan Assay overcomes the challenges in assay-to-assay variability experienced by antibody-based platforms, with its industry-leading CVs that are maintained as the platform scales.
| The SomaScan Assay uniquely scales with 6x better precision than HP antibody assay |
||||
|---|---|---|---|---|
| SomaScan 11K Assay | SomaScan 5K Assay | High-plex antibody assay 1* | High-plex antibody assay 2* | |
| # Proteins | 11,083 | 5,284 | 5,420 | 3,072 |
| Median CV | 6.8% | 6.6% | 35.7% | 19.8% |
| *Source: Rooney, M.R. et al., 2024. Plasma proteomic comparisons change as coverage expands for SomaLogic and Olink. medRXiv. | ||||
| The SomaScan Assay uniquely scales with 6x better precision than HP antibody assay | |||
|---|---|---|---|
| SomaScan 11K Assay | SomaScan 5K Assay | ||
| 11,083 Proteins | 5,284 Proteins | ||
| 6.8% Median CV | 6.6% Median CV | ||
| High-plex antibody assay 1* | High-plex antibody assay 2* | ||
| 5,420 Proteins | 3,072 Proteins | ||
| 35.7% Median CV | 19.8% Median CV | ||
| *Source: Rooney, M.R. et al., 2024. Plasma proteomic comparisons change as coverage expands for SomaLogic and Olink. medRXiv. | |||
PUBLICATION
Rooney, M.R. et al.
Plasma proteomic comparisons change as coverage expands for SomaLogic and Olink.
medRxiv (2024)
The study tested the precision of the newest versions of high-plex proteomic assays, assessing the reproducibility of two affinity-based platforms using split plasma samples from 102 Atherosclerosis Risk in Communities (ARIC) Study participants. Results showed that the SomaScan 11K Assay had a median CV of 6.8% (vs 6.6% for the subset of assays available on the SomaScan 5K Assay) versus a median CV of 35.7% (vs 19.8% for the subset of smaller assays) for another platform, indicating that the high-plex antibody platform has CVs that are almost 6x larger than the SomaScan Platform.
SomaScan Assay has superior consistency in precision across the entire platform coverage.
- The majority of protein measurements using the SomaScan 11k Assay show a CV <10%.
- Protein measurements using a high-plex antibody assay show variable CVs, with nearly half around 50%, as indicated by the spike on the right.
- The SomaScan Assay effectively scales protein measurements while other technologies cannot.
2024 Nature corrections: The SomaScan Platform leads in precision and number of proteins.
The corrected Nature article comparing the performance of SomaScan Assay vs. high-plex antibody assays shows that the SomaScan Platform leads in precision and number of proteins. This underscores the significance of precise CV calculations for reliable scientific findings and improved outcomes in population proteomics studies using the SomaScan Assay.
PUBLICATION
Eldjarn, GH et al.
Large-scale plasma proteomics comparisons through genetics and disease associations.
Nature 622, 348–358 (2023)
Comparison of two affinity-based platforms was performed using biobank data from the UK Biobank generated by the UKB Pharma Proteomics Project and from Iceland. Analysis was stratified on ancestry and uncovered genomic sequence variants associated with plasma protein levels (protein quantitative trait loci (pQTLs)) and biomarkers of diseases and their progression. Precision measurements between the assays were calculated using CV. Across all assays, the Somascan Assay consistently demonstrated lower CVs of 9.9% and 9.5% versus 16.5% and 14.7%, respectively.
Read another viewpoint
PUBLICATION
Candia, J et al.
Variability of 7K and 11K SomaScan plasma proteomics assays
bioRxiv (2024)
This study evaluates the precision and reproducibility of the SomaScan Platform, which has been upgraded from the 7K to the 11K version. The research builds on the previous assessments of the 7K Assay by analyzing technical replicates from the Baltimore Longitudinal Study of Aging.
Join the leading-edge of proteomics
Given the SomaScan Platform’s unparalleled scalable reliability and precision, it has been employed as the leading proteomic platform for clinical trial studies by top pharmaceutical companies such as Bristol Myers Squibb1, Novo Nordisk2, Gilead3 and Pfizer4 in a range of therapeutic areas including cardiometabolic disease, oncology, and neurodegeneration.
With the SomaScan 11K Assay, you gain precision, stability and comprehensive coverage to drive meaningful discoveries in proteomics. Trust your data as your proteomics content grows and achieve unparalleled insights into protein biomarkers for improved research outcomes.
Discover how the
SomaScan 11K Assay can improve your research
REFERENCES
- Brown, E. A. et al. Effect of pegbelfermin on NASH and fibrosis-related biomarkers and correlation with histological response in the FALCON 1 trial. JHEP Reports, Volume 5, Issue 4, 100661
- Schattenberg, J. et al. Prevalence of, and effect of semaglutide on, features of non-alcoholic steatohepatitis in patients with obesity with and without type 2 diabetes: analysis of data from two randomised placebo-controlled trials using SomaSignal tests. J.Hepatol., 78(S1), S811-S812.
- Kowdley et al. EASL June 2023
- Sivakumar, P. et al. SomaLogic proteomics reveals new biomarkers and provides mechanistic, clinical insights into Acetyl coA Carboxylase (ACC) inhibition in Non-alcoholic Steatohepatitis (NASH). Scientific Reports, 14, Article 17072




