Latest research shows benefit of non-invasive, high-plex protein profiling for liver disease
Pharma researchers use non-invasive SomaSignal® tests to advance understanding and treatment strategies for liver disease and NASH
Exciting new discoveries have been made recently by researchers using the NASH bundle of SomaSignal® tests as a non-invasive, liquid biopsy approach to identify novel protein biomarkers related to liver disease and non-alcoholic steatohepatitis (NASH). See below for recent research from our pharma partners. Want to discuss these results or learn how high-plex protein profiling could accelerate your research? Contact us today.
Correlation of a nonalcoholic steatohepatitis proteomic test with clinical outcomes
Anne Minnich, PhD, biomarker consultant at Bristol Myers Squibb, presented published research on the use of the SomaSignal NASH bundle in the recently completed FALCON 1 Phase IIb multicenter trial investigating the use of three pegbelfermin doses in patients with NASH.
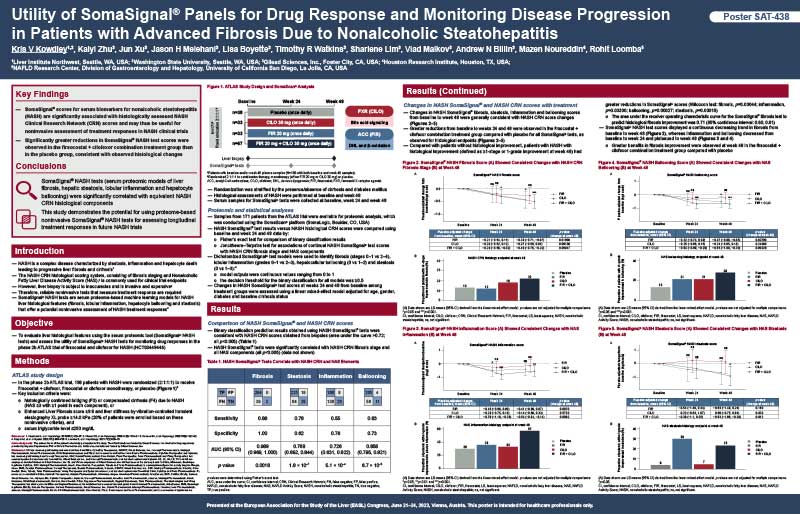
Utility of SomaSignal panels for drug response and monitoring disease progression in patients with advanced fibrosis due to nonalcoholic steatohepatitis
Researchers from Liver Institute Northwest, Washington State University, Gilead Sciences, Houston Research Institute, and NAFLD Research Center – University of California San Diego presented results from the Phase IIb ATLAS trial that showed SomaSignal test scores for NASH are significantly associated with histologically assessed NASH Clinical Research Network (CRN) scores and may thus be useful for noninvasive assessment of treatment responses in NASH clinical trials.
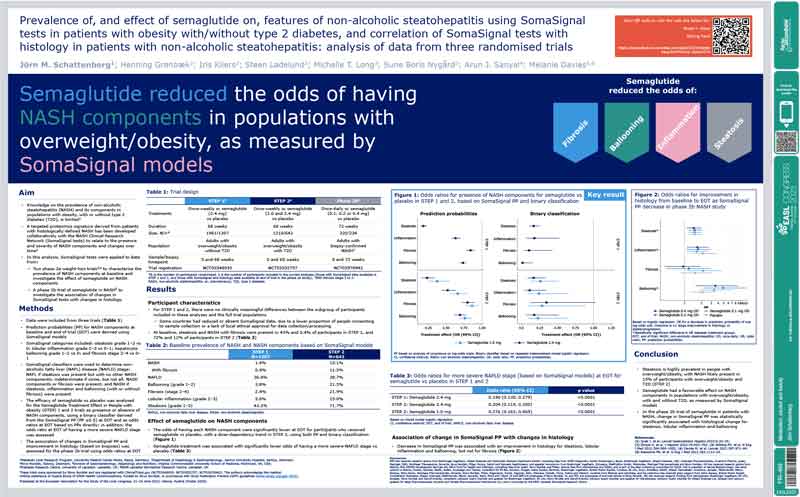
Semaglutide reduced the odds of having NASH components in populations with overweight/obesity, as measured by SomaSignal models
Researchers from University Medical Center Mainz, Aarhus University Hospital, Novo Nordisk, Virginia Commonwealth University, Diabetes Research Centre – University of Leicester, and NIHR Leicester Biomedical Research Centre applied data from two Phase IIIa weight loss trials and one Phase IIb trial and found semaglutide had a favorable effect on NASH components in patients with overweight/obesity, with and without Type 2 diabetes.
Want to learn more about SomaSignal tests for pharma research?
Our SomaSignal tests are based on a dataset of ~3 million protein measurements that we developed by analyzing thousands of patients using our high-plex, high-throughput SomaScan Assay. Learn more about the NASH bundle and all our SomaSignal tests here.
Want to know more about using high-plex, aptamer-based protein profiling for NASH research?
See more research, webinars, and our full SomaSignal test portfolio here. And watch our video to see how our proprietary SOMAmer® (Slow Off-rate Modified Aptamer) protein affinity reagents work.
Want a quick update
when we publish new
NASH-related research?
More posters
PosterOptimizing biomarker discovery with focus on low coefficient of variation in large-scale proteomics
Coefficients of variation (CV) describe innate technical variation in high throughput molecular measurement platforms and are a standard metric for characterizing and monitoring assay precision. Median CVs range from ~4.5% to 18.0% for immunoassay technology, 1 up to >30% for mass spectrometry,2 ~5% for the SomaScan® Assay, and ~10% for the Olink Explore Assay (Figure 1). Large CVs can cause technical variability to overwhelm biological signal.
PosterA proteomic predictor of conversion from mild cognitive impairment to dementia with potential utility in enhancing productivity of emerging clinical trials
A significant proportion of individuals with mild cognitive impairment (MCI) develop dementia, with annual conversion rates exceeding 10%. Earlier dementia diagnosis and intervention can improve outcomes, and new disease-modifying drugs are being repositioned for the preclinical stages of illness.
PosterComparison of Proteomic CV Risk to Established ASCVD 10-Year Risk Decision Points
The ASCVD pooled cohort equation (PCE) is well-established for CV risk assessment. Decision points for determining treatment plans are low, intermediate and high risk over 10 years, however this approach over and underestimates risk in certain subgroups. The validated CV Risk SomaSignal® Test (SST) provides 4-year risk probability of MACE allowing for timely assessment of risk, but the shorter timescale makes comparison to 10-year PCE risk less intuitive.






