Blood proteins reveal biological age of human organs to help track health and disease
How the human plasma proteome changes with age
Have you ever heard someone say, “But I feel 10 years younger!”? This common expression highlights the difference between how we feel and the number that defines our age. Our numerical age is linear – a universal reference point to indicate the passage of time – but does not necessarily reflect health status.
The field of aging research seeks to understand the physiological processes that influence the condition of organs, tissues and cells over the human lifespan. While chronological age is simply a number, biological age can be influenced by genetic, lifestyle and environmental factors, and may be an accurate predictor of physiology1.
Technologies to estimate biological age are continually being developed and refined with the overarching goal of better understanding the primary drivers of age-related chronic disease and mortality.
Taking a proteomics-based approach to studying aging can be powerful, as plasma protein levels change rapidly in response to the main influences of biological age. Measuring proteins over the course of a lifetime, across a large number of individuals, provides a picture of how the proteome shifts throughout a “normal” lifespan and can serve as a reference point for the process of aging.
How proteomics can decipher the aging trajectory
Researchers in the lab of Tony Wyss-Coray, PhD, at Stanford University study aging and its effects on the human body. Understanding how our bodies – and even specific organs – change molecularly with age could help address the global disease burden associated with aging and support the development of preventive medicine and improved patient care.
The SomaScan® Platform is one of the tools used by the lab, which uses modified DNA aptamers (SOMAmer® Reagents) to bind target proteins with high specificity, enabling the assessment of thousands of human proteins in plasma.
The team leveraged high-plex SomaScan Technology to uncover punctuated changes in the human plasma proteome associated with age. They discovered that these changes, particularly involving cell signaling2, occur in a nonlinear fashion at three distinct timepoints when humans are in their 40s, 70s and 80s.
This landmark study has been replicated across a wide variety of cohorts (and species2) and has transformed the way we view the aging trajectory. Findings have been cited nearly 400 times in other high-impact journals and the paper has 54,000 downloads!
The work has provided foundational information about how humans respond to external stressors and change as we age. Building on this earlier research, Wyss-Coray’s team then published novel findings featured as the cover article in Nature (December 2023): Organ aging signatures in the plasma proteome track health and disease3.
In an effort to provide insight into common age-related changes in the proteome, the team again used the SomaScan Assay to quantify organ-specific plasma proteins from blood samples to noninvasively assess organ health over time and characterize thousands of healthy individuals across their lifespan.
Based on a new set of proteomic data, the team found that individual organs appear to be on unique aging trajectories distinct from other organs and from the organism as a whole3. This groundbreaking research showed that a substantial portion of “healthy” adults have one or more organs with accelerated aging, and this confers a greater risk of earlier mortality even when there are no noticeable symptoms of disease.
Using plasma proteins to determine organ age gaps
The researchers first identified organ-enriched gene sets for 11 organs that have well-understood contributions to age-related disease. Then, plasma proteins measured by the SomaScan 7K Assay were mapped to each of the organs to generate an organ-specific proteomic signature.
By using independent cohorts of healthy individuals aged 20–90 years, changes in these proteomic organ signatures could be characterized across the lifespan. Organ “age gaps,” a measure of an individual’s organ biological age relative to other same-aged peers, were calculated for each organ within each individual.
1 in 5 adults have
an accelerated aging signature
Results revealed for the first time that nearly 1 in 5 seemingly healthy adults have one or more organs with an accelerated aging proteomic signature (accelerated aging was defined as a one standard deviation higher organ age compared with the group average organ age among same-aged peers). Patients who had no indicators of disease at the start of the study but had an accelerated heart aging proteomic profile had a 250% increased risk of heart failure, and a 150% increased risk of all-cause mortality within 15 years of follow-up.
Similarly, the team linked 202 plasma proteins to accelerated aging of the brain and from those created a model that predicted cognitive decline. Strikingly, this organ-specific signature predicted cognitive decline as strongly as phosphorylated Tau (pTau-181), which is currently the best blood biomarker of Alzheimer’s disease risk.
Moreover, these organ-age signatures were significant across all five independent cohorts in the study, reflecting strong replication across datasets. Two of the cohorts were run on the SomaScan 5K Assay and three on the SomaScan 7K Assay, which highlights an important feature of SomaScan Technology: Data from different platform versions can be combined seamlessly without needing to run bridge samples.
When the organ aging models developed by the Wyss-Coray team are divided into either secreted or non-secreted protein groups, they can be further identified as occurring in low abundance vs. higher abundance (Figure 1).
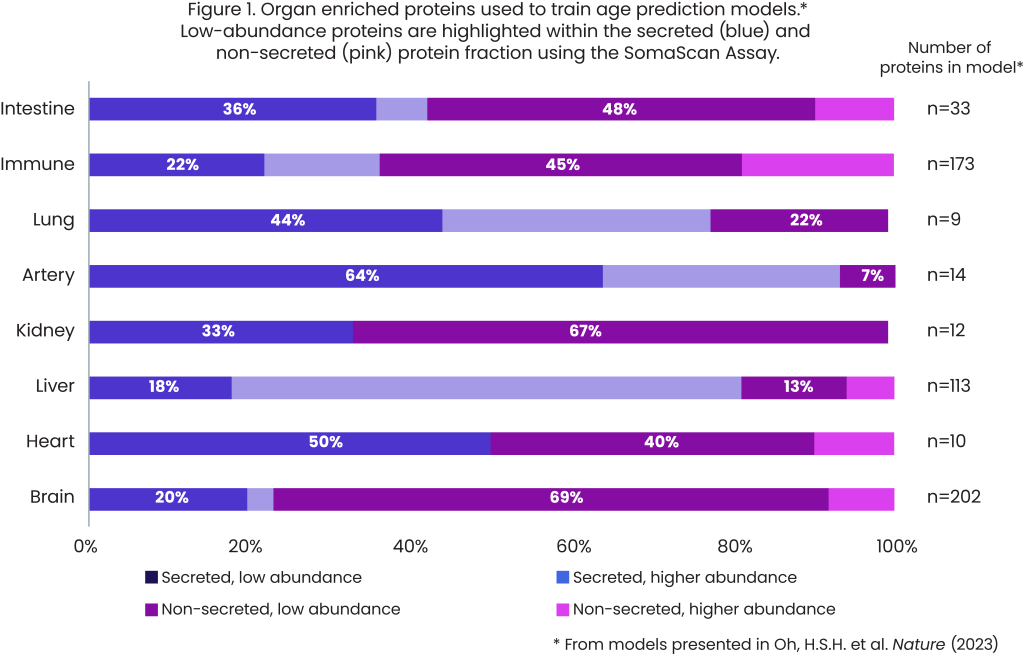
The brain organ aging model was trained on 202 plasma proteins: 156 (77%) are non-secreted proteins and 140 (69%) are low-abundance, non-secreted proteins. This means that the large majority of plasma proteins associated with brain aging are non-secreted and present in low abundance. The SomaScan Platform is unique in that it can reliably measure this fraction of the proteome to uncover biologically relevant signals of aging.
Uncover biologically relevant signals of aging with the SomaScan Assay
Wyss-Coray believes the impact of this research will be far-reaching: “If we can reproduce these findings in 50,000 or 100,000 individuals, it will mean that by monitoring the health of individual organs in apparently healthy people, we might be able to find organs that are undergoing accelerated aging in people’s bodies, and we might be able to treat them before they get sick4.”
Indeed, the power of measuring thousands of proteins in large cohorts of individuals has exciting implications for human health. The SomaScan 11K Assay offers even more opportunity for discovery of protein biomarkers indicating significant physiological milestones and mechanisms controlling the aging process and enables detection of proteins across all major age-related disease areas and biological pathways. The assay measures half of the human proteome, including low-abundance, non-secreted proteins previously thought to be unimportant, but now known to be otherwise.
The brain organ aging model was trained on 202 plasma proteins: 156 (77%) are non-secreted proteins and 140 (69%) are low-abundance, non-secreted proteins. This means that the large majority of plasma proteins associated with brain aging are non-secreted and present in low abundance. The SomaScan Platform is unique in that it can reliably measure this fraction of the proteome to uncover biologically relevant signals of aging.
Uncover biologically relevant signals of aging with the SomaScan Assay
Wyss-Coray believes the impact of this research will be far-reaching: “If we can reproduce these findings in 50,000 or 100,000 individuals, it will mean that by monitoring the health of individual organs in apparently healthy people, we might be able to find organs that are undergoing accelerated aging in people’s bodies, and we might be able to treat them before they get sick4.”
Indeed, the power of measuring thousands of proteins in large cohorts of individuals has exciting implications for human health. The SomaScan 11K Assay offers even more opportunity for discovery of protein biomarkers indicating significant physiological milestones and mechanisms controlling the aging process and enables detection of proteins across all major age-related disease areas and biological pathways. The assay measures half of the human proteome, including low-abundance, non-secreted proteins previously thought to be unimportant, but now known to be otherwise.
While we all may intuitively understand the difference between chronological and biological age, pioneering research led by Wyss-Coray has identified proteomic signatures of organ aging that are distinct from whole-body aging and can predict early disease and mortality.
Listen to Wyss-Coray present this exciting research, along with other ongoing work on aging, in this recent webinar: Young Blood for Old Brains and the Quest to Slow Brain Aging
To learn more about how the SomaScan Assay can enable discoveries in aging research, see this white paper: Identify Protein Biomarkers for Aging Research With the SomaScan Assay
References
- Baker III, G.T. and Sprott, R.L. “Biomarkers of aging.” Experimental Gerontology 23 (1988): 223–239.
- Lehallier, B. et al. “Undulating changes in human plasma proteome profiles across the lifespan.” Nature Medicine 25 (2019): 1,843–1,850.
- Oh, H.S.H. et al. “Organ aging signatures in the plasma proteome track health and disease.” Nature 624 (2023): 164–172.
- Goldman, B. “Stanford Medicine-led study finds way to predict which of our organs will fail first.” Stanford University (2023): accessed May 2024.
More blogs
BlogHow Blood Proteomics Is Accelerating Neuro Drug Discovery
Explore how researchers are using blood-based proteomics – including data from the Global Neurodegeneration Proteomics Consortium (GNPC) – to uncover new protein signatures, genetic risk associations and mechanistic insights into aging and neurodegeneration.
BlogGNPC Neurodegeneration Proteomics Dataset
On July 15, 2025, the Global Neurodegeneration Proteomics Consortium (GNPC) published four open‑access papers unveiling the largest disease‑specific proteomic resource ever assembled.
BlogIndependent study validates the SomaScan Assay as the most precise and comprehensive plasma proteomic platform
A recent independent study by Alkahest, published as a preprint on bioRxiv1, compared the technical precision and performance of multiple plasma proteomic platforms using a common set of samples.





