Advancing rare disease research with powerful protein profiling
Uncover clues to disease progression, and assess
therapy effectiveness for the world’s most unique diseases.
The SomaScan® Assay offers new insights into rare disease biology and biomarker discovery by profiling 11,000 protein measurements in every sample
With more than 300 million people living with a rare disease worldwide,1 helping to conquer the unique challenges presented by rare diseases is a passion shared by everyone at SomaLogic. Delivering accurate information and mapping the journey forward for researchers, patients, and caregivers begins with data—the kind of targeted data the SomaScan Assay can provide.
Learn how researchers use the SomaScan Assay to enrich their rare disease studies
In these recent presentations, researchers share the discoveries they’ve made, as well as the impact of using high-plex protein profiling to study glioblastoma and idiopathic multicentric Castleman disease (iMCD).
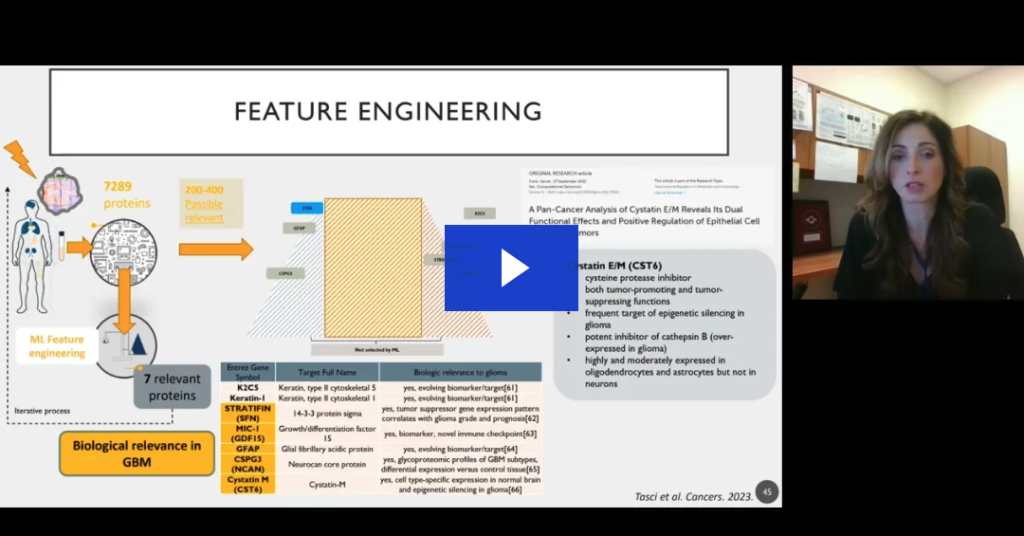
Harnessing AI and proteomics for glioblastoma
Andra Krauze, MD, Physician Early Investigator and radiation oncologist at NCI/NIH, explains how her team uses high-plex protein profiling with the SomaScan Assay to for unlocking the biocomplexity of glioblastoma and create effective therapeutic strategies in the clinic.
Accelerating research and treatment for Castleman Disease
In addition to his rare disease research credentials at University of Pennsylvania, Dr. Fajgenbaum is also a patient battling idiopathic multicentric Castleman disease (iMCD). He became ill during his third year of medical school in 2010…
SomaScan Assay protein profiling for biomarker discovery assists in understanding rare disease progression and assessing response to therapy
The SomaScan Assay has been used to study a variety of rare diseases.
See below for highlights of 3 specific studies.
Duchenne Muscular Dystrophy (DMD)
DMD is a rare genetic disease that causes progressive muscle weakness and degeneration. It occurs primarily in males, though 8%-10% of female carriers are also affected. Most children with DMD use a wheelchair by their early teen years. Treatments are available to slow the progression of the disease, but there is no cure.2
Understanding DMD at the protein level
- SomaScan technology was used to screen for protein biomarkers associated with DMD using serum samples from two independent cohorts.
- The SomaScan Assay identified 44 circulating serum biomarkers associated with patients with DMD vs the healthy control group.
- Data suggest new protein targets and biomarkers for further DMD studies and may facilitate future clinical studies to identify new therapeutics for DMD.
- The SomaScan Assay may offer a path forward for research and clinical experts in DMD to pursue potential improvements in clinical trial designs, and to generate new diagnostic and therapeutic approaches to this devastating disease.
Cystic Fibrosis (CF)
CF is a hereditary lung disease caused by a defect in the cystic fibrosis transmembrane conductance regulator (CFTR) protein, a membrane glycoprotein that governs ion flux at the surface of epithelial cells. Although treatments have been developed to improve CFTR protein function, lung disease persists. A better understanding of disease pathogenesis and prognosis can help pave the way for improved therapies.3
Optimizing biomarker discovery for CF
- In order to understand the lung proteome, it is necessary to study a complex mixture of thousands of interacting proteins present in a large range of concentrations.
- Samples from 50 patients with CF were studied using the SomaScan Assay and 1,129 proteins from each sample were quantified.
- SomaScan Assay analysis revealed a complex pulmonary environment with many protein candidates that may aid in assessing this challenging disease and developing new therapeutic targets.
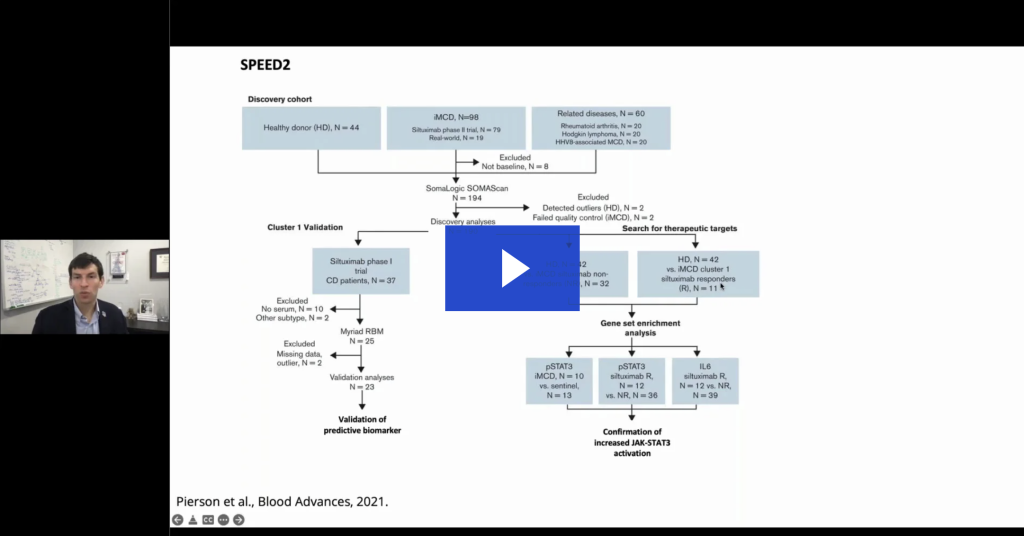
Uncovering new targets for treating idiopathic multicentric Castleman disease (iMCD)
iMCD is a poorly understood hematologic disorder involving cytokine-induced polyclonal lymphoproliferation, systemic inflammation, and potentially fatal multiorgan failure. The annual incidence of the disease is approximately 1500 individuals in the United States and the 5-year overall mortality rate is 35%-45%. Siltuximab is the only FDA-approved treatment for Castleman disease, but not everyone responds to it.4
Serum protein profiling for iMCD—a personalized medicine approach
- In the discovery cohort, the SomaScan Assay was used to measure 1305 serum analytes using DNA-based aptamer technology.
- Serum proteomic quantification was performed in samples of patients with iMCD to define disease classification, discover predictive biomarkers of response, and identify treatment targets for Siltuximab nonresponders.
- The data identified and validated a novel subgroup of patients with iMCD with superior response to Siltuximab and uncovered candidate therapeutic targets for nonresponders, addressing 2 major unmet medical needs.
Deepen your molecular insights of new biomarkers and therapeutic targets with the SomaScan Assay – now capable of 11,000 protein measurements.
That’s HALF the genetically encoded human proteome!
Learn more
See peer-reviewed, published studies from researchers worldwide using the SomaScan Assay
References:
- Nguengang Wakap S, Lambert DM, Olry A, et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. 2020;28(2):165-173. doi:10.1038/s41431-019-0508-0
- Hathout Y, Brody E, Clemens PR, et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2015;112(23):7153-7158. doi:10.1073/pnas.1507719112.
- DeBoer EM, Wagner BD, Popler J, et al. Novel application of aptamer proteomic analysis in cystic fibrosis bronchoalveolar lavage fluid. Proteomics Clin Appl. 2019;13(3):e1800085. doi:10.1002/prca.201800085.
- Pierson SK, Shenoy S, Oromendia AB, et al. Discovery and validation of a novel subgroup and therapeutic target in idiopathic multicentric Castleman disease. Blood Adv. 2021;5(17):3445-3456. doi:10.1182/bloodadvances.2020004016.
Contact one of our experts to learn more
The statements herein have not been evaluated by the Food and Drug Administration.