![]()
The BEST Reliability in High-Throughput Proteomics
Measure high- and low-abundance proteins confidently with superior sensitivity, high specificity, and proven reproducibility.
![]()
High-Plex Proteomics Services and Experts Near You
Outsource your high-throughput screening to our fee-for-service, CLIA-certified, CAP-accredited lab at U.S. World Headquarters, or to our worldwide network of SomaLogic Authorized Sites.
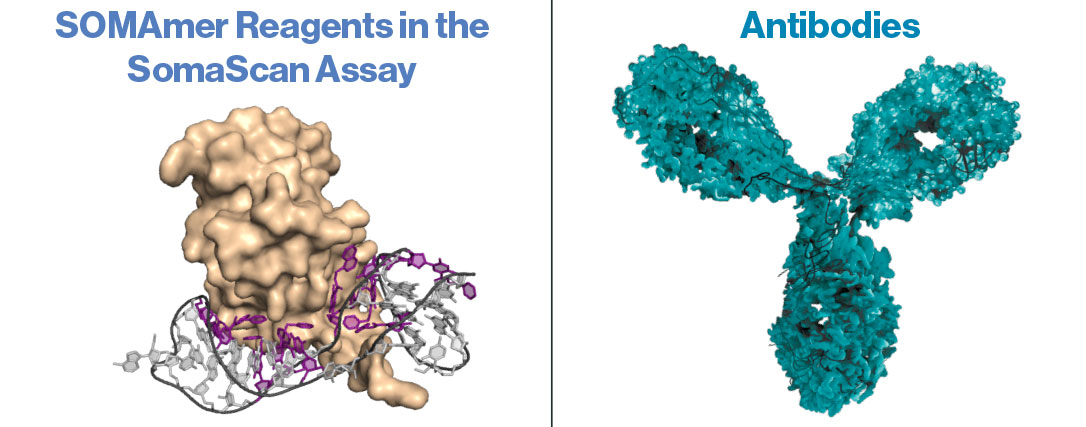
Aptamers vs. Antibodies
How are SOMAmer® Reagents different from antibodies for affinity-based protein assays?
Explore the power of more
![]()
More proteins
Gain targeted insights and make better decisions with thousands of protein measurements from a 55-µL sample.
![]()
More pathways
Get broad, deep coverage – half the human proteome – in a number of therapeutic areas on a scalable platform.
![]()
More pQTLs
Be confident in your results
with our SOMAmer® technology’s industry-leading reproducibility, sensitivity, and specificity.
![]()
More proof
See success in action with 800+ peer-reviewed publications, 1,000+ issued and pending patents, and 3+ billion proteomic measurements and counting.
One platform for discovery, validation, and development
Move from discovery to validation quickly and efficiently on a single platform. Whether you are a scientist in an academic medical center or at a pharmaceutical, biotechnology, or contract research organization, we can help you collaborate at the intersections of basic research, preclinical and potential clinical applications in a wide range of therapeutic areas.
With our pioneering technology, you’ll get high sample throughput with a low sample volume for a variety of sample types, so you can be confident in your results.
Learn how you can gain new biological insights into the human proteome and the complex molecular interactions of human health and disease with the SomaLogic solutions listed below.
Not sure how to choose?
Therapeutic areas
Cardiovascular disease
Discover multiple cardiovascular indicators from a 55-µL plasma sample.
Neurology
Get a high-throughput, multiplex proteomics solution that focuses on the complexity of neurological diseases.
COVID-19
With thousands of protein measurements, you can find patterns no one knew to look for.
Diabetes
The SomaScan® Assay could advise patients on lifestyle changes to help prevent the onset of type 2 diabetes.
Rare disease
Advancing rare disease research with powerful protein profiling and offering new insights into biomarker discovery.
Cancer
Accelerate your biomarker discovery with sensitive detection of thousands of protein measurements from a 55-µL sample.




